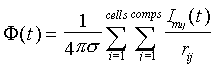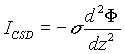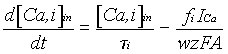Our model is built using three levels: supracellular (field potentials
and CSDs obtained from the synchronous activity of a population of neurons),
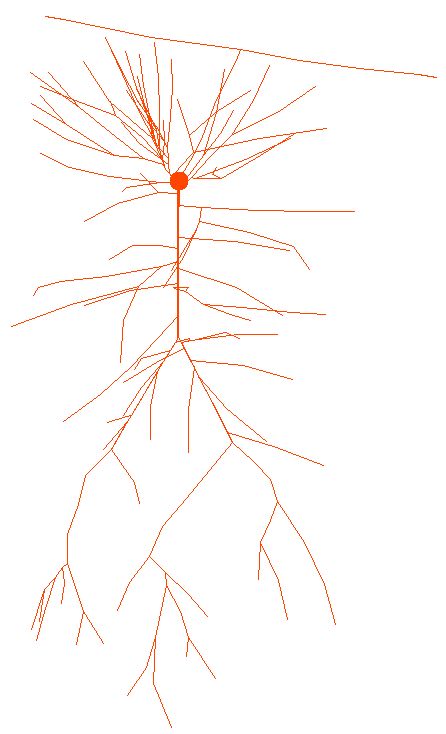
cellular (morphology and physiology of the single neurons that make up
the ensemble) and subcellular (electrotonic properties, ionic active channels
and calcium dynamics for each locus in the morphology). The dorsal CA1
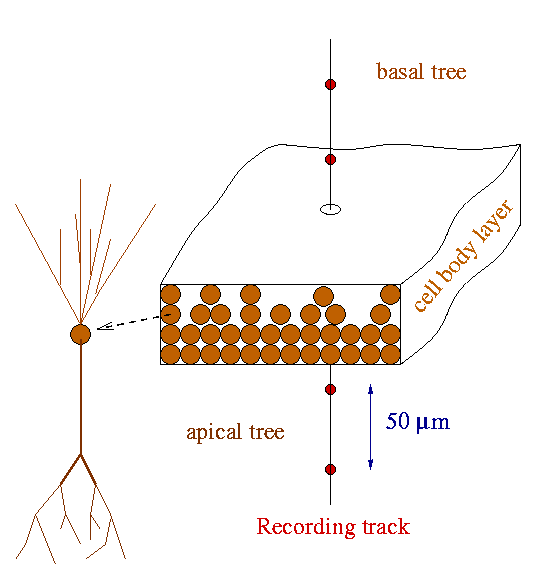
region was modeled with aggregates of different size preserving an experimentally
observed cell density of 64 neurons in a 50x50 µm lattice (Boss
et al., 1987). The antero-posterior and latero-medial dimensions of
the aggregates were 0.05x0.05, 0.2x0.2, 1x0.35, 1x1, and 3x2 mm, corresponding
to 64, 1,024, 6,072, 17,424 and 104,544 morphologically identical model
neurons, respectively. The dimensions and cell number of the largest aggregate
can be taken as a rough estimation for the actual values for the dorsal
CA1 region. Three different spatial distribution of neurons were made with
their somata arranged either in a monolayer, 4 layers of even density,
or a realistic distribution of 4 uneven layers with 66% of somata in the
apical side and 22 and 11% in the two layers of the basal side (see schemes
in Figure 3 of the paper). Each neuron was rotated a random angle around
the somatodendritic axis to test that the particular morphology used in
our experiments introduced no artifacts in the field potential calculations.
The curvatures in the dorsal CA1 region were neglected for this study.